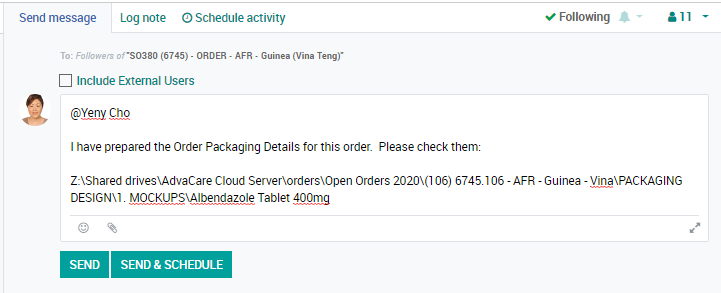
Getting started with an OPD (Order Packaging Details)
The Order Packaging Details document provides the factory with very detailed information required by the distributor of the product, packaging and design according to their market demands. This document is to be prepared by the Salesperson for all pharmaceutical, veterinary, and supplement products of each order once we have received the down payment for the order.
Note: the OPDs must be prepared by the moment when Purchasing Specialist is signing the Purchase Order with the vendor as OPD becomes a part of the Purchase Order which facilitates the vendor to follow the stated requirements
OPD Types
The Order Packaging Details Document could be created in 2 ways depending on the situation or records of the ordering products...
• New Product for Distributor:
1) Prepare a new Order Packaging Details document- If this is a new product we have not produced before, Salesperson will need to prepare a new Order Packaging Details document.
2) Order Packaging Details for the same product has been prepared for another distributor- If this product has been produced before for another distributor, Salesperson can reference the Order Packaging Details document from another distributor. Only need to edit according to the distributor’s requirements.
• Re-ordered Product for Distributor:
1) Re-Ordered Product with NO Order Packaging Details- If Salesperson does not find the Order Packaging Details for a re-ordered product, Salesperson must prepare a new Order Packaging Details document.
2) Re-Ordered Product with YES Order Packaging Details- If Salesperson finds the Order Packaging Details document in the design folder, copy and paste the document into the new order’s product folder of the PACKAGING DESIGN folder in MOCKUP folder. This information must be included in the “Packaging Requirements” section of each product in the SO.
How to create an Order Packaging Details for NEW PRODUCT
Salesperson needs to prepare the Order Packaging Details document for a new product according to the distributor’s requirements.
There are 2 ways to prepare the Order Packaging Details document if it has not been prepared previously for the distributor: 1) Order Packaging Details for the same product has been prepared for another distributor 2) Prepare Order Packaging Details document for new product
1) Order Packaging Details for the same product has been prepared for another distributor
1. Search the design folder to check if this product has ever been ordered before by any other distributor and if there is an Order Packaging Details document already prepared. Check all specifications are the same as for your order.
Z:\Shared drives\AdvaCare Cloud Server\design\"category folder"\"form folder"\"product folder"\"product dosage folder"
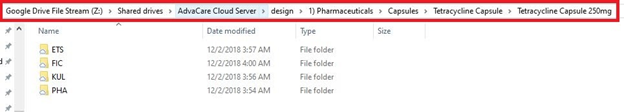
Example: Z:\Shared drives\AdvaCare Cloud Server\design\1) Pharmaceuticals\Capsules\Tetracycline Capsule\Tetracycline Capsule 250mg
2. Find the most recent order by checking each distributor folder and see if the Order Packaging Details document has been prepared for that order. If so, check that the specifications are the same as your order. If not, edit the Order Packaging Details as required. Do NOT edit the document for the other client. Copy and paste to the PACKAGING DESIGN folder of your order.
2) Prepare a new Order Packaging Details document
If this is a new product we have not produced before, Salesperson will need to prepare a new Order Packaging Details document.
1. Copy the Order Packaging Details template according to the form of the product.
Z:\Shared drives\AdvaCare Cloud Server\sales\ORDER PACKAGING DETAILS (Templates)
2. Paste into the product folder of the order under PACKAGING DESIGN FOLDER
Z:\Shared drives\AdvaCare Cloud Server\orders\"Open Orders XXXX (YEAR) folder"\"order #” folder\PACKAGING DESIGN\1. MOCKUPS\"product”folder
Example: Ferrous Sulfate + Folic Acid Tablet, 10 tablets/blister, 10 blisters/box
3. Revise the yellow highlighted sections according to the products specifications.

4. To complete the Packaging requirements: section, salesperson must inform purchasing to get the details from the factory. If the distributor has specific requirements, a task should be sent to purchasing to confirm if the factory can produce as per distributor’s requirements.
1) Packing specification: XX tablets/(XX type)blister, (XX type)blisters/box, XX boxes/shrink wrap, XX boxes/carton (1 manual per box)
(If there is a middle box, must be included here)
2) Tablet information: (Salesperson sends task to purchasing to ask factory to send us a photo of what the tablet will look like) This is very important as the design of the tablet will be on the box so the information must be accurate as per the actual tablet.
• Color: what is the exact color of the tablet(product)? There are many shades of a color so we need to get as close to the actual color for the tablet design on the box.
• Score in the middle: does the tablet have a line to split the tablet?
• Print on tablet: does the tablet have any printing? For example, some factories stamp “P500” on the tablet for Paracetamol tablet 500mg. But this will depend if the factory already has a mould for the stamp.
• Coated (film-coated) or Uncoated?: Is the tablet coated or uncoated? If it is coated, the tablet should always be film-coated. Never sugar-coated.
If the product’s form is not tablet, the related to the form specifications will vary. Salesperson must clarify on all points that are mentioned in the OPDD to avoid any confusion for the vendor, as they must know what they have to produce precisely.
3) Blister information: PVC or Alu Alu blister, size, blister background color
• PVC blister: Clear, green, red? This is the front part of the blister. Generally it is clear, but there may be a few products that require a different color PVC.
• Size: XXxXXmm (check blister design for size)
• Blister background color: white (should always be white)
• Print on blister: Batch number, manufacturing date, expiration date, registration number? There are some distributors which have this information printed on the blister if the product has been registered like this. Also, some distributors require the registration number on the designs if the product is registered. Salesperson can check the past orders to check on this.

Or
• Stamp on blister: batch number and exp. date.
Generally, the batch number and expiry date will be stamped on each blister if there are no requirements.

As there will not be a finished product picture for a new product, Salesperson should attach the blister design from our MOCKUP.
4) Box:
• Box quality: 350g white-white cardboard, Aqueous Coating (this information changes depending on the product’s packaging size/product’s range)
1) Standard box for pharmaceutical products with the packaging less than 1,000 tablets/box = 350g white-white cardboard, Aqueous Coating
2) Standard box for pharmaceutical products with the packaging more than 1,000 tablets/box = 350g white-white cardboard, film-coated (glossy);
3) Standard box for supplement products with the packaging more than 1,000 tablets/box = 400g of white cardboard, film-coated (glossy);
For detailed information refer to PACKAGING SPECIFICATION GLOSSARY
• Packing form: explain how blisters are packed in the box. (attach a photo from another product)
Ex: Put 5 blisters(vertical)/row, 2 rows/box, the insert is placed in the box

5) Carton:
Brown five-layer corrugated paper export carton, blue printing, design and carton size keep the same with 2 PP packing belts (打包带)
(this information does not change unless the distributor requires white cartons)
6) Packing requirements:
Put 2 kraft paper pads inside the carton, one on the bottom and one on the top, check the packaging and seal with adhesive tape.
(this information does not change)
Always send a task to the Sales Manager to check the order packaging details when completed.
How to create an Order Packaging Details Document for RE-ORDERED PRODUCT
1. Check if the product has been ordered before, open the ALL PRODUCTS ORDERED excel sheet in the OTHERS folder:
Z:\Shared drives\AdvaCare Distributors Cloud Server\"distributor folder"\OTHERS
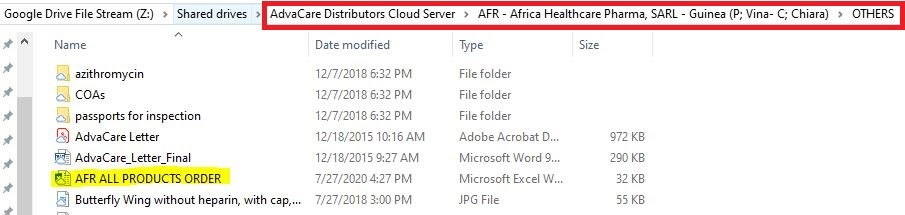
2. Open the excel sheet and search for the product and the last order this product was ordered. Be sure that the FORM, DOSAGE and PACKAGING are the same, not only the product name.
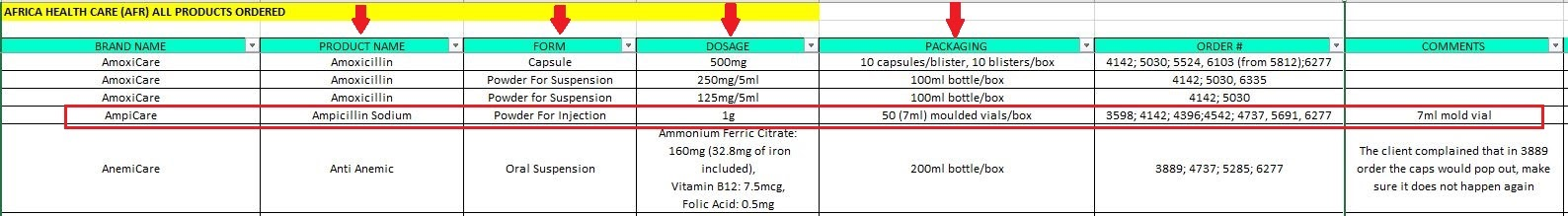
3. Open the design folder and follow the path according to the product specifications:
Z:\Shared drives\AdvaCare Cloud Server\design\"category folder"\"form folder"\"product folder"\"product dosage folder"\"distributor folder"
Example: Z:\Shared drives\AdvaCare Cloud Server\design\1) Pharmaceuticals\Powder for Injection\Ampicillin Sodium Powder for Injection\Ampicillin Powder for Injection 1g\AFR
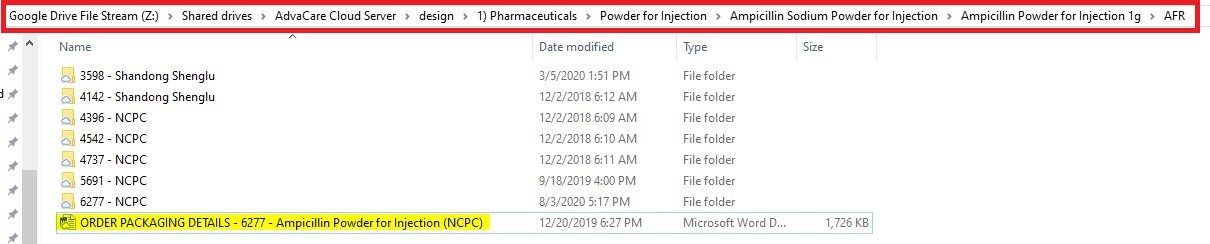
4. Copy and paste the Order Packaging Details from the design folder to the current order’s PACKAGING DESIGN folder under the product name.
Open the word document and edit:
1. Order #
2. Order #-FACTORY CODE-Date
3. Quantities (quantities and cartons will change per order)

4. All other information must be thoroughly checked as per the Customer’s requirements.
5. Send a task to the Sales Manager to check the completed Order Packaging Details documents for the order before sending the task to Purchasing to send to the factory.
Include the link of where the document is saved: