When registration (site/product) is completed, generally a certificate is granted by the MOH to the Customer.
• Site Registration Certificate (SRC): certificate that states a manufacturing site has been registered in the MOH system and therefore import of products from this manufacturing site will be permitted. Most countries may require further product registration before allowing any imports, while some countries may allow just a simple “product notice” application to start allowing product imports from the manufacturing site.
• Certificate of Product Registration (CPR): certificate that states a product produced by a manufacturer has been registered in the MOH system and therefore import of products from this manufacturing site/marketing authorization holder will be permitted.
Customer has to share the copy of this certificate as a proof of the registration status. When the SRC/CPRs are shared by the Customer, BD Salesperson must complete these steps to record this information in the system.
SAVING THE SRC/CPR IN SERVER FOLDER
The SRC/CPRs must be saved in the following locations, and under the following formats:
1. Distributor Folder: Registration Certificates
Z:\Shared drives\AdvaCare Distributors Control\”Distributor Details”)\REGISTRATION DOCUMENTS\REGISTRATION CERTIFICATES
1.1 Create a folder “SOXXXX” with the Registration SO#.
1.2 Save the Certificate(s) under the name format:
CPR: “Product Brand/Generic Name” + “Form” + “Dosage” - “Vendor”
SRC: “Vendor Name” Site Registration Certificate
2. Manufacturer Folder: Registration Certificates
China: Z:\Shared drives\AdvaCare Cloud Server\Manufacturers\Manufacturers\”Vendor Details”\Registration Certificates
India: Z:\Shared drives\AdvaCare Purchase India Control\AdvaCare Purchasing\Manufacturers\Manufacturers\”Vendor Details”\Registration Certificates
2.1 Create a folder “CUSTOMER CODE - Registration Country” with the 3 letter internal Code.
2.2 Save the Certificate(s) under the name format:
CPR: “SOXXXX” - “Product Brand/Generic Name” + “Form” + “Dosage”
SRC: “SOXXXX” Site Registration Certificate
3. Registration Control - Countries - Registration Certificates
Z:\Shared drives\AdvaCare Registration Control\Countries\”Country”\REGISTRATION CERTIFICATES
3.1 Create a folder “CUSTOMER CODE” with the 3 letter internal Code.
3.2 Save the Certificate(s) under the name format:
CPR: “SOXXXX” - “Product Brand/Generic Name” + “Form” + “Dosage”
SRC: “Vendor Name” Site Registration Certificate
UPDATING THE REGISTRATION MILESTONE & SENDING TASK
1. Open the registration SO Project.
2. Update the Registration Milestone: REG: 15. Registration Certificates Received
3. Send a task to VP of Sales, BD Dept. Manager, Account Manager (for ordering distributors), and Sales Dept. Manager (for ordering distributors) to inform that registration has been completed with the product list and link to the Distributor Registration Certificates folder.
UPDATING PR/RA AND CREATING SRC/CPR IN THE REGISTRATION MODULE
Product Registration
1. Open the PR page of the received product registration.
2. Click “GENERATE CPR”.
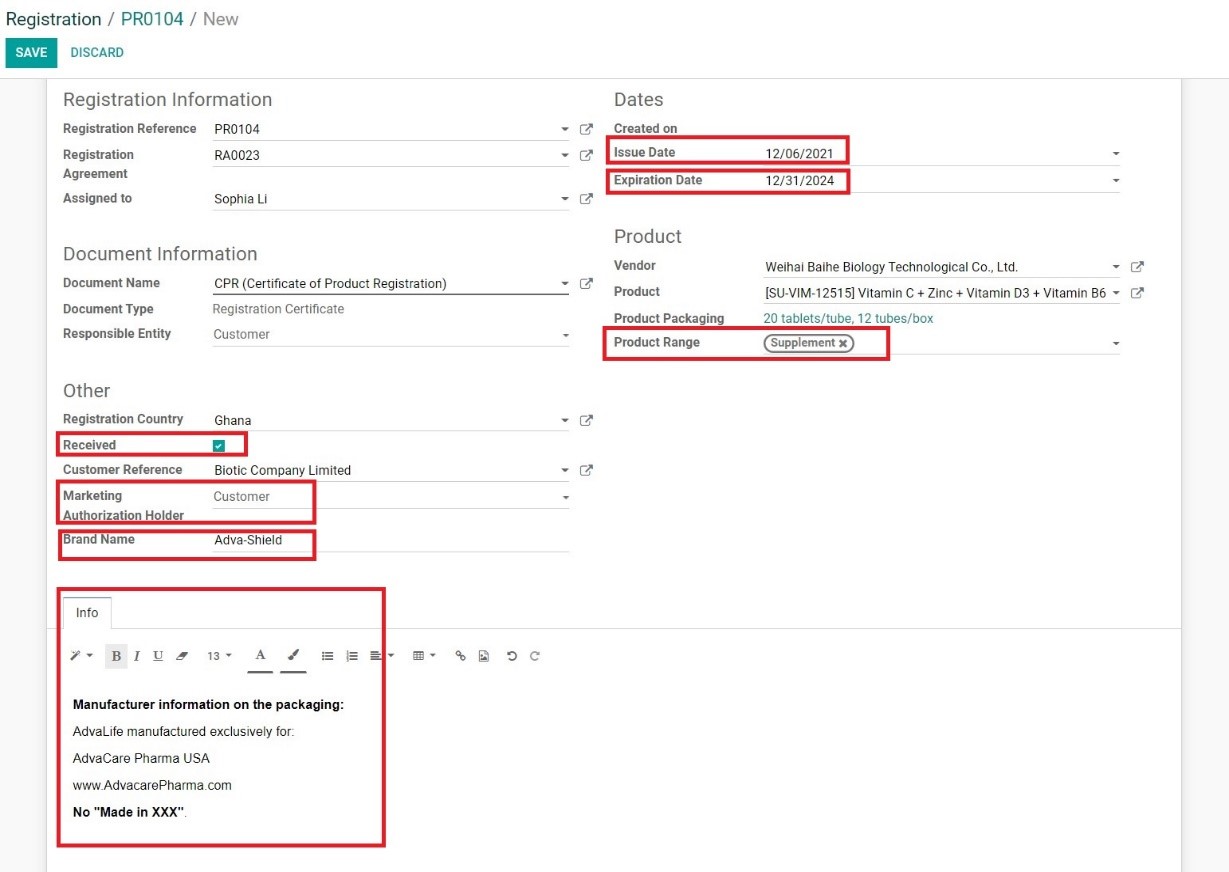
3. A newly created CPR page will be opened. Fill in the information as follows:
• Issue Date: input the date official date CPR was issued as per the certificate
• Expiration Date: input the expiration date of CPR as per the certificate
• Product Range: type and select the relevant product range
• Received: tick if registration certificate is saved in the server
• Marketing Authorization Holder: select the MAH as per shown on the certificate
• Brand Name: input the registered product brand name
• Info: add additional information either from the certificate or packaging submitted and approved (manufacturing name/address requirements, etc.)
4. Click “SAVE”.
5. Change the CPR pipeline status to “COMPLETE”.
6. Use the breadcrumbs to return to the PR page.
7. Change the PR pipeline status to “COMPLETED”.
8. Add VP of Sales, BD Dept. Manager, Purchasing Dept. Manager, Account Manager (if applicable) and Sales Dept. Manager (if applicable) as followers to the newly created CPR.
Site Registration
1. Open the RA page of the received product registration.
2. Click “EDIT”.
3. Input the “End Date” to the date of registration completion.
4. Change the RA pipeline status to “COMPLETED”.
5. Click “SAVE”.
6. Go to the Documents - Documents page.
7. Click “CREATE”.
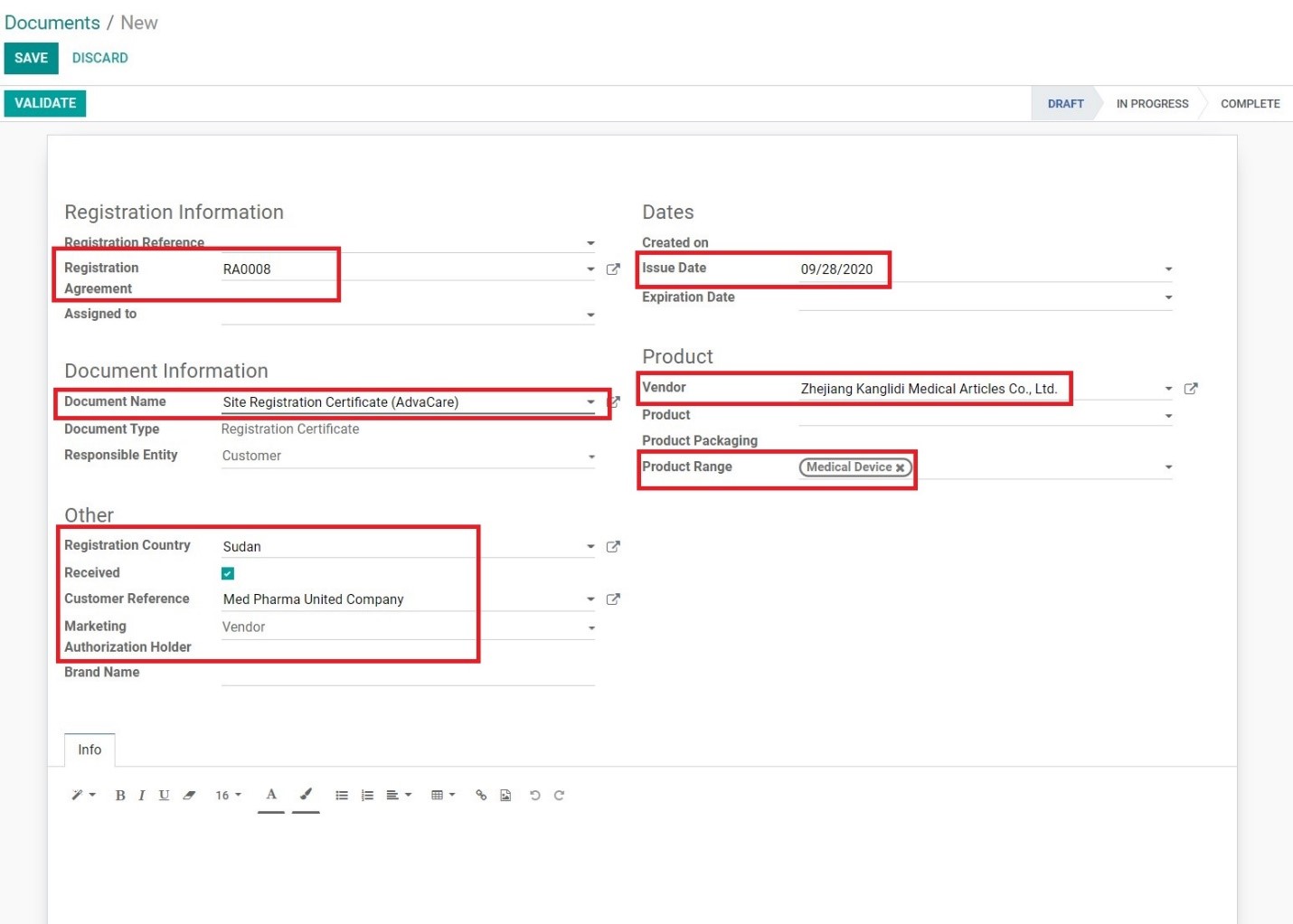
8. A newly created Documents page will be opened. Fill in the information as follows:
• Registration Agreement: type and select the RA# of the site registration
• Document Name: type and select “Site Registration Certificate (AdvaCare)”
• Registration Country: type and select the registration country
• Received: tick if registration certificate is saved in the server
• Customer Reference: type and select the Customer, best to add by Internal Code
• Marketing Authorization Holder: select the MAH as per shown on the certificate (usually Vendor)
• Issue Date: input the date official date SRC was issued as per the certificate
• Expiration Date: input the expiration date of SRC as per the certificate (if applicable)
• Vendor: type and select the Vendor registered
• Product Range: type and select the relevant product range under the SRC
• Marketing Authorization Holder: select the MAH as per shown on the certificate
• Info: add additional information as applicable, usually information that will be shown on the packaging of products for an order.