Registration Documents Database
| 6 minutesDocuments refer to a database of documents for registration of products in a country.
Getting started with Documents Database
Under the “Registration Module”, a dropdown will be shown when you hover over the top button “Documents”.
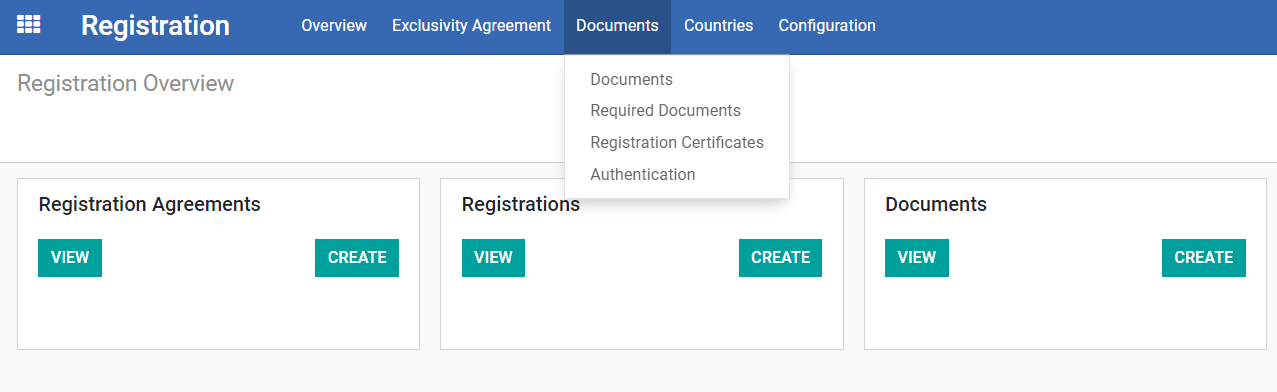
The drop down includes the following, and intended for as a Master List of:
1) Documents
All documents that exist in our system, it lists the documents that a Vendor can provide and/or documents that have been provided to a Customer in a registration. The table under Documents will have the following fields:
China Registrations:
Z:\Shared drives\AdvaCare Cloud Server\Manufacturers\Manufacturers\(Vendor Name)\Certificates
Z:\Shared drives\AdvaCare Distributors Cloud Server\(Customer Code) - (Customer Name) - (Country) - (Salespersons)\REGISTRATION DOCUMENTS\SOXXX\Final
India Registrations:
Z:\Shared drives\AdvaCare Purchase India Control\AdvaCare Purchasing\Manufacturers\Manufacturers\(Vendor Name)\Certificates
Z:\Shared drives\AdvaCare Purchase India Control\AdvaCare Purchasing\Registration\(SOXXX) - (Customer Code) - (Country) - (Salesperson)\REGISTRATION DOCUMENTS\Final
2) Required Documents
All documents any Country MOH may require. The documents under this list are to be added to Customer contact cards and their Registration RA based on each Customer requirements. The table under Required Documents will have the following fields:
3) Registration Certificates
All completed registrations under AdvaCare with any Country MOH. In the system, a registration certificate is called CPR (Certificate of Product Registration). The table under Registration Certificates will have the following fields (* meaning there is only one option):
Z:\Shared drives\AdvaCare Distributors Cloud Server\(Customer Code) - (Customer Name) - (Country) - (Salespersons)\REGISTRATION DOCUMENTS\Registration Certificate\SOXXX
Z:\Shared drives\AdvaCare Cloud Server\Manufacturers\Manufacturers\(Vendor Name)\Registration Certificates
Z:\Shared drives\AdvaCare Registration Control\Countries\(Country)\Registration Certificates
4) Authentication
All document authentications (legalization/notarization) any particular Country MOH may require. The documents under this list are to be added to Customer contact cards and their Registration RA based on each Customer requirements.. The table under Authentication will have the following fields:
Using the Documents Database
The following provides standard instruction to use the Documents database and its main objectives.
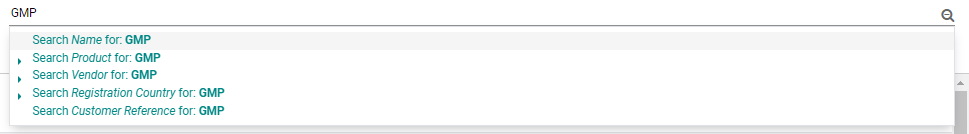
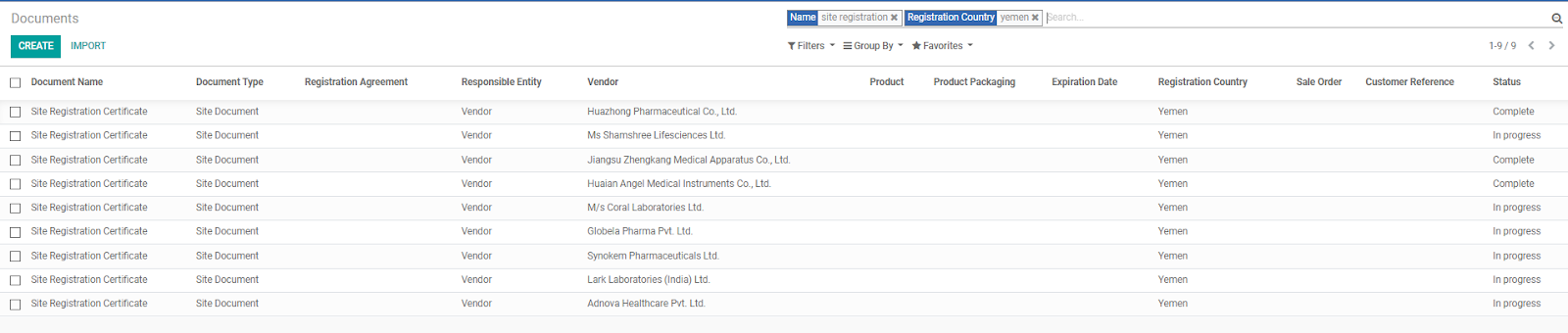
Some useful functionalities of the Documents database includes:
• Checking possible Vendors for registration in a Country
Some countries have strict requirements in which physical site inspections by the MOH are required before registration can be completed, or a reference country (stricter country) MOH registration must be completed. BD Salesperson/Purchasing Dept. can check the database to find compatible Vendors for better efficiency when working on an RFQ.
• Checking if Required Product Document has been obtained from previous registration
Some technical documents such as SmPC (Summary of Product Characteristics) contain basic information of a product from a vendor that can be reused with minimal revisions. Rather than asking for the same documents multiple times from the same vendor, RA Dept. can first check the database if it has been received previously.
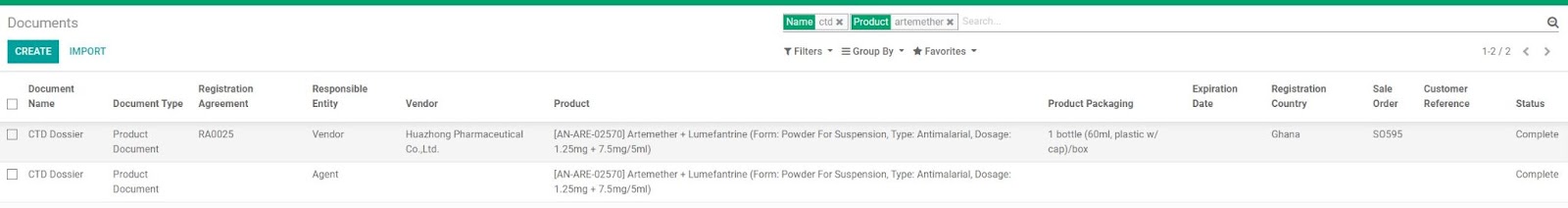
• Checking if a Product Document required from an agent has been previously purchased
Some documents such as CTD Dossier, BE Studies can be reused for different registrations even with different Vendors. Before purchasing a new required product document from the Agent, BD Salesperson/RA Dept. can check whether these documents have existed before.
Using the Registration Certificate Database
The following provides standard instruction to use the Registration Certificate database and its main objectives.
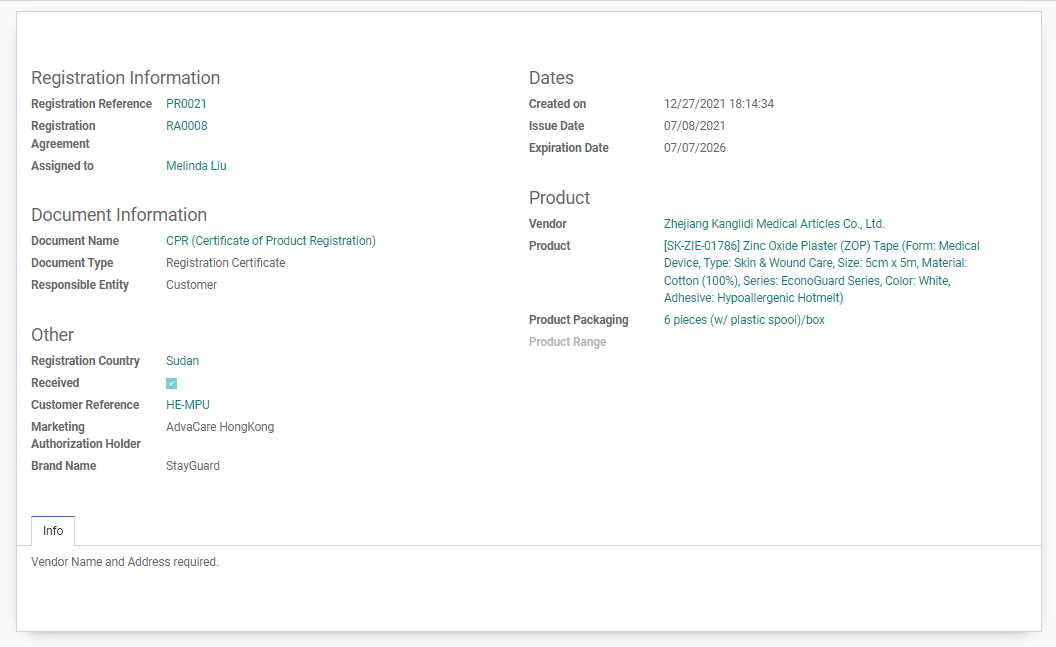
Some useful functionalities of the Registration Certificate database includes:
After product registration completion, Customer will start an order of the product. When working on an RFQ, Purchasing/BD Salesperson/AM Salesperson can check all information relevant to the registration including Vendor, Packaging, Brand Name, and additional Info for the packaging.