Registration Countries Database
| 4 minutesCountries refer to a database of information relevant to each country’s Distribution/Registration requirements, capabilities and/or limitations. This database serves the purpose of providing detailed and accurate information for Salespersons to handle each country inquiry tailored to its own requirements.
Getting started with Countries Database
Open the Registration Module.
Click on the “Countries” button on the top.
A list of countries will be shown under a table when the top button “Countries” is clicked. A quick overview of this table shows the following:
Click on a Country to view more information relevant only to that country, or use the search bar on the top right of the screen by inputting the Country Name and pressing “Enter”.
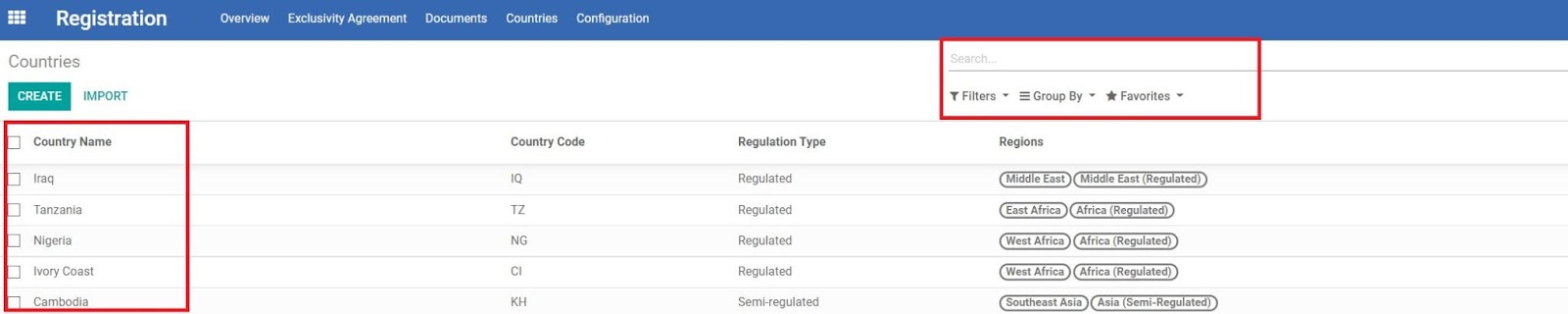
In each Country, more information can be found for this country. From top to bottom, there are some important quick links/tabs.
TOP (QUICK LINKS)
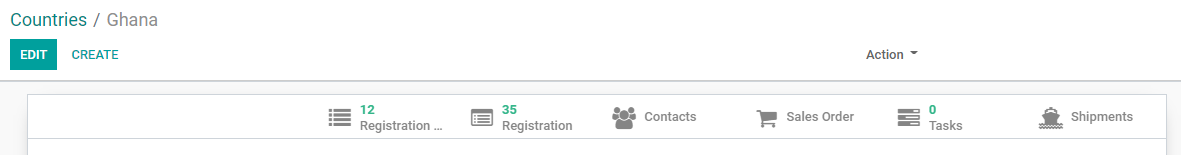
• Registration...
Links to existing Registration Agreements (RA) for this country. Click the tab to see a table listing all RA(s) with more information.
• Registration
Links to existing Product Registration (PR) for this country. Click the tab to see a table listing all PR(s) with more information.
• Contacts
• Sales Order
• Tasks
Links to all tasks for a REGISTRATION, RFQs or ORDERs that require registration in this country.
• Shipments
MAIN PART (DESCRIPTION)
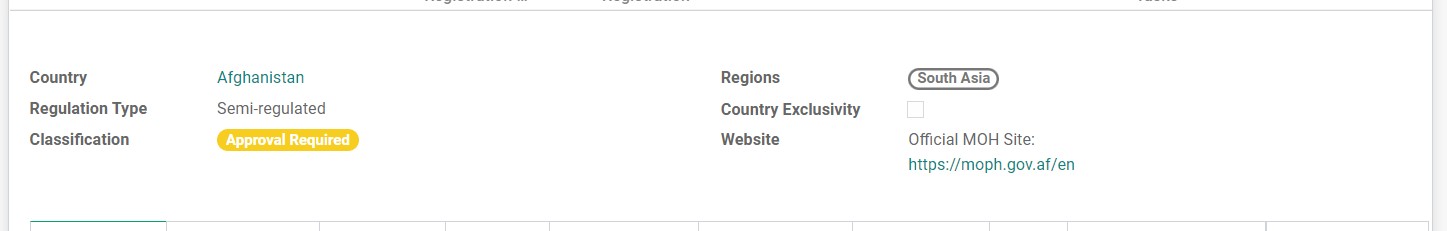
• Country
• Regions
Each country will have at least two region tags. One is to show the geographical region of the country, and another is to classify the country in the same group with other countries from a larger geographical region but with similar regulatory requirements.
• Regulation Type
There are three types of Regulation Type based on the registration requirements for Pharmaceutical product in this country:
• Country Exclusivity
The checkbox will be ticked if there is a Country-Enforced exclusivity that gives limitations of registration in this country.
• Classification
There are several types of Classification of country, this information is based on the majority of inquiries received and may change over time.
Depending on the Classification of the country, there are different ways for how the inquiry from this country is to be handled.
• Website
Links to official MOH sites where information about registration can be found.
• Text field
The text field provides a link where users can find a more detailed regulatory guideline and relevant forms in our system for each country.
BOTTOM (TABS)
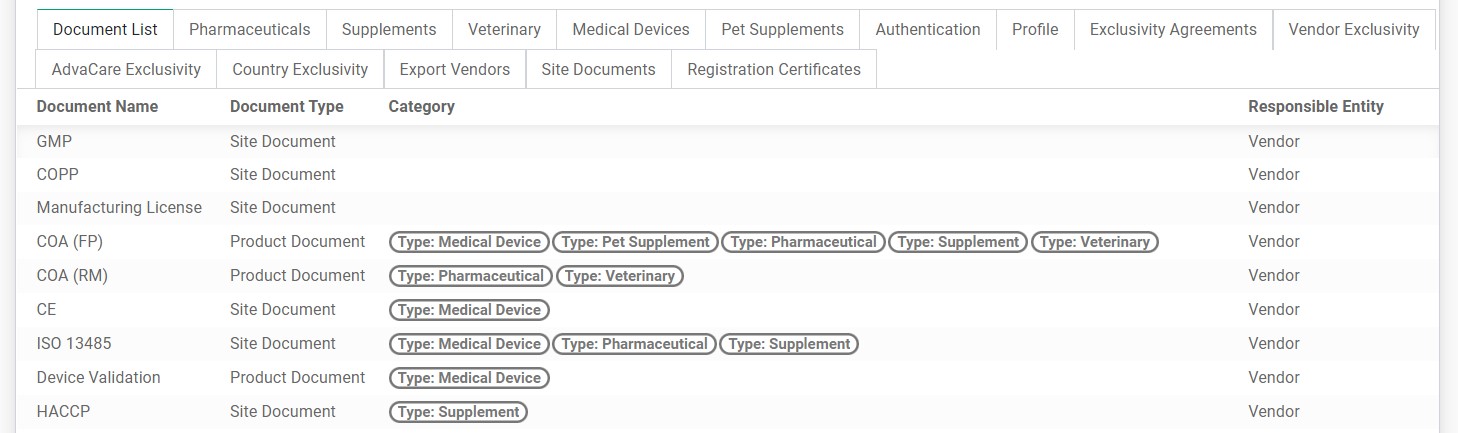
• Document List
A list of documents required for registration in this country. It lists all ranges that are verified for this country.
• Pharmaceuticals / Supplements / Veterinary / Medical Devices / Pet Supplements
A list of generalized documents required for registration of each product range in this country.
• Authentication
A list of document authentication (legalization/notarization) required for registration in this country. It lists all ranges that are verified for this country. To get the separated list of legalized documents by range, follow the folder link on the text field above.
• Profile
Provides some background information for Salespersons to better handle each country inquiry. It includes: ECONOMIC BACKGROUND, MARKET BEHAVIOR, PROSPECT DYNAMICS, REGULATORY BODY, and MORE INFORMATION.
• Exclusivity Agreements
A list of all Exclusivity Agreements in the system together with the state of each exclusivity.
• Vendor Exclusivity
A list of vendor exclusivity that provides limitations in working with certain vendors for this country.
• AdvaCare Exclusivity
A list of exclusivity granted by AdvaCare to customers in this country.
• Country Exclusivity
A list of Country-Enforced exclusivity that gives limitations of registration in this country.
• Export Vendors
A list of vendors that have exported to this country, regardless of registration status.
• Site Documents
A list of vendors that have obtained approval of this country’s specific GMP requirement.
• Registration Certificates
A list of all AdvaCare’s completed registrations in this country.
Country Related Discussions
The different aspects of a country in terms of regulatory requirements, exclusivity, economic situation, etc. can affect how specific country inquiry is to be handled. This information is constantly changing and developing. In order to ensure that any updates to one country are informed through the relevant departments, all communications that are COUNTRY related are to be handled under the Country chatter.