In a product registration, packaging design is one of the key requirements for submission required for:
1. Submission of packaging design to the MOH.
2. Samples submission.
It is important that the packaging design reflects the product information accurately prior to submitting to the MOH, while maintaining the company key design requirements. Packaging design for registration normally consists of the following elements:
• Primary packaging: blister/pouch/bag/label/tube design/ampoule print
• Secondary packaging: box/bag/label
• Insert (if applicable)
Process of packaging confirmation is complex and long. The process will be different for China Registration vs. India Registration. Follow these processes to handle the packaging process:
PACKAGING DESIGN - CHINA REGISTRATION
The basic steps of confirming packaging design for China Registration includes:
I. Confirming Packaging Requirements from the Customer.
II. Checking and Confirming Mockup Designs.
II. Sending Packaging Design to the Customer and signing Packaging Design Confirmation Agreement (PDCA).
IV. Confirming Packaging Design with the Vendors & Signing “Final Packaging”.
I. Confirming Packaging Requirements from the Customer
1. In the first process of starting registration, BD Salesperson must have already confirmed with the Customer the basic requirements of the packaging design with the Customer including whether or not the following will be require:
• Vendor Name/Address
• “Made in XXX”
2. If the Customer has shared any registration guidelines, check to make sure if packaging design requirements are mentioned to double confirm each requirement. Some countries may have additional requirements such as:
• Importer Name/Address.
• Product brand name not according to AdvaCare format.
• Registration No. field to be added for the registration number after approval.
• Use of local language on all/parts of the packaging design.
3. Additional step to obtain approval will be required for these cases:
• Importer Name/Address: get approval from VP of Sales and confirmation with Purchasing Dept. that the selected Vendor will be suitable for this.
• Product brand name not according to AdvaCare format: get approval from VP of Sales and Marketing Dept.
4. Once the requirements are clear and confirmed, provide all of this information to the Design Dept. via task.
II. Checking and Confirming Mockup Designs
1. Design Dept. will communicate with Vendors directly on the physical specifications of the packaging, such as box size, bottle size, etc., and prepare the packaging design mockup as per task. Design Dept. will send a task to BD Salesperson when the mockup design has been completed.
2. BD Salesperson will check the mockup design to make sure the following details are correct on every single part and pieces of the packaging design:
• Product brand name, generic name, dosage.
• MOH requirements. confirmed in the previous step such as Vendor Name/Address, etc.
• Product information (indication, administration method, storage conditions, etc.) written on the packaging design is correct. If not sure, BD Salesperson can refer to existing packaging designs to compare. For new products never produced before, BD Salesperson must refer to external references as well (Google).
• Secondary packaging shape, size and orientation makes sense to AdvaCare’s standard packaging requirements.
• Packaging Specification of the products such as materials of primary and secondary packaging are consistent with AdvaCare’s standard packaging requirements.
Refer to the REFERENCE SOP: Packaging Design Checklist &
Packaging Specification Glossary
for further information on AdvaCare’s standard format of packaging design.
3. For Pharmaceutical, Veterinary, and some Medical Device products, BD Salesperson must create/revise the product inserts (leaflet).
Refer to the REFERENCE SOP:
Insert Templates
for further information on AdvaCare’s standard format of product insert.
4. While checking the mockup design, BD Salesperson creates the Batch Number excel sheet that can be found in each Customer’s packaging design folder. It is saved in the following link for each Customer:
Z:\Shared drives\AdvaCare Distributors Cloud Server\"Client Code"\PACKAGING DESIGN\"Registration Number"
5. Edit the name of the Batch Number excel sheet to this format:
6. Open the file and input the SO#, product names and dosages in the registration under the first column.
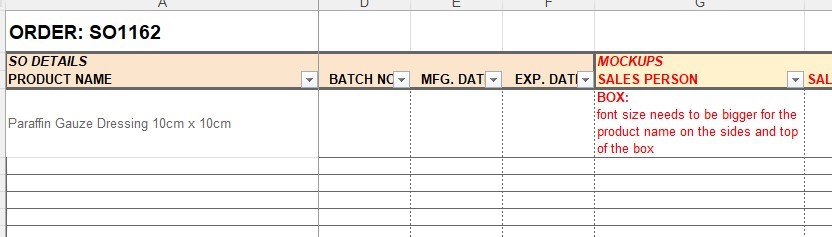
7. Any comments for Design Dept. to make revisions of the mockup design must be written under the “MOCKUPS - SALES PERSON” column.
5. Edit the template as per each component of each product and attach the MOCKUP designs accordingly.
6. Make sure that Customer’s signature field is present at the end of every page of the PDCA. Save the PDCA in the following location:
Distributor Folder → PACKAGING DESIGN
7. Send the PDCA to the Customer via email and ask them to sign/stamp each and every page of the PDCA.
8. Once the signed PDCA has been received, set the Registration Milestone Date for REG: 6. Customer Confirms Packaging save in the PACKAGING DESIGN folder with the name format:
IV. Confirming Packaging Design with the Vendors & Signing “Final Packaging”
In an order process, mockup packaging design must be sent to the Vendors for production. This also applies for registration samples that Vendors will be responsible for printing. However, even if sample packaging is NOT required from the Vendor, it is important that the Vendor confirms the mockup design that will be submitted for registration, as some information may be inconsistent in the design vs. the actual products that will be produced by Vendor in the future or even the registration documents that are being prepared by the Vendor.
1. After mockup design has been finalized, send a task to Design Dept. to send the mockups to the Vendors.
2. Vendors will check the packaging design and specifications. Generally, Vendors will send back their “Factory Packaging”.
3. BD Salesperson must check and compare factory packaging with the mockup packaging.
4. If there are any discrepancies that the Vendor must revise, BD Salesperson must write the comments on the Batch Number excel sheet under the FACTORY PACKAGING - SALES PERSON column.
5. After checking and recording all the revision requirements in the excel sheet, BD Salesperson will send task for other departments to check depending on the Customer status:
• Customers already assigned to an Account Manager: Salesperson will send a task to the assigned Account Manager to check the Factory Packaging and record their comments in the excel sheet.
• Customers that are yet assigned to an Account Manager: Salesperson will send a task to BD Dept. Manager to check the Factory Packaging and record their comments in the excel sheet.
6. If factory packaging mistakes are made from the Vendor side: Once feedback from Account Manager/BD Dept. Manager has been received, send a task to Design Dept. to ask Vendors to make these revisions.
7. After revisions are made, Design Dept. will send a task with the new sets of factory packaging. BD Salesperson checks and makes sure that all the comments are fixed.
8. After the final factory design has been confirmed by BD Salesperson, send a task to Account Manager and BD Dept. Manager to recheck and provide the final confirmation.
9. If factory packaging requests revisions on our mockup: If revisions required from the Vendors are something to be revised in the mockup, repeat from the previous stage II and revise the packaging mockup.
10. Once factory packaging has been finalized, print out in color every part of each product’s factory packaging. BD Salesperson must write the SO# and date of confirmation on the packaging, sign every page, and provide the printed copies to Design Dept. BD Salesperson must also set the Registration Milestone Date for REG: 7. Manufacturer Confirms Packaging.
11. Design Dept. will scan these copies called “FINAL PACKAGING” and send them to the Vendors as a form of confirmation.
FOR VETERINARY PRODUCTS
Veterinary product packaging presents an additional layer in preparing packaging as the details such as intended animals, indication, dosage, withdrawal period and other information are very broad and may vary Vendor to Vendor. For this product range, it is best that BD Salesperson confirms with the Vendor that information on the packaging will be consistent with the REGISTRATION DOCUMENTS that Vendors are working on.
PACKAGING DESIGN - INDIA REGISTRATION
The basic steps of confirming packaging design for China Registration includes:
I. Confirming Packaging Requirements from the Customer.
II. Getting Packaging Details from Vendors.
III. Checking and Confirming Mockup Designs.
IV. Confirming Packaging Design with the Vendors.
V. Sending Packaging Design to the Customer and signing Packaging Design Confirmation Agreement (PDCA).
VI. Confirming & Signing Final Packaging with the Vendors.
I. Confirming Packaging Requirements from the Customer
This step is the same as the above for PACKAGING DESIGN- CHINA REGISTRATION - STEP I .
II. Getting Packaging Details from Vendors
1. In the beginning of the registration process, BD Salesperson must have asked India Purchasing Person to obtain the following information from the Vendors:
• PP & draft COPP.
• Vendor Packaging Mockup.
• Product Insert.
• Pictures of the finished product (tablet pictures, bottle pictures, box pictures etc.).
2. Follow up with the Purchasing Person if this information has not been received.
3. Once received, check the information of the PP and draft COPP to make sure they are consistent.
4. If other parts of the packaging in the above list cannot be provided by the Vendor, ask the questions that will provide information about the packaging details and specifications. BD Salesperson can use the Request for Packaging Component Details template to be sent to the Vendor to fill in. This template can be found in
REFERENCE SOP:
Request for Packaging Component Details
. Once filled in and sent back by the Vendor, save the template under the
PACKAGING DESIGN - FACTORY PACKAGING folder.
5. If PP and COPP are consistent, and other details of the packaging components obtained, BD Salesperson can proceed to inform the Design Dept. to proceed with preparing a mockup based on the information provided by the Vendor together with the Customer’s requirements.
III. Checking and Confirming Mockup Design
This step is the same as the above for PACKAGING DESIGN- CHINA REGISTRATION - STEP II, except for step No. 4 where the location of packaging design will be under this location instead:
Z:\Shared drives\AdvaCare Purchase India Control\AdvaCare Purchasing\Registration\”Registration Number and Details”\PACKAGING DESIGN
IV. Confirming Packaging Design with the Vendors
India packaging has stricter requirements to be followed than China packaging. Therefore, before confirming packaging with the Customer, it is best to first confirm that the main parts of a packaging design related to regulatory requirements are first confirmed by the Vendor.
1. Make sure that the inserts have been prepared in CDR/JPG format by Design Dept. based on the word/pdf format prepared by BD Salesperson.
2. After mockup design has been finalized internally, send a task to Design Dept. to lock the CDR files of all mockup designs.
3. Send a task to Purchasing Dept. to send the locked CDRs to the Vendors.
4. Vendors will check the packaging design and specifications. Generally, Vendors will send back their “Factory Packaging”.
5. BD Salesperson must check and compare factory packaging with the mockup packaging.
6. If there are any discrepancies that the Vendor must revise, BD Salesperson must write the comments on the Batch Number excel sheet under the FACTORY PACKAGING - SALES PERSON column.
7. After checking and recording all the revision requirements in the excel sheet, BD Salesperson will send task for other departments to check depending on the Customer status:
• Customers already assigned to an Account Manager: Salesperson will send a task to the assigned Account Manager to check the Factory Packaging and record their comments in the excel sheet.
• Customers that are yet assigned to an Account Manager: Salesperson will send a task to BD Dept. Manager to check the Factory Packagingand record their comments in the excel sheet.
8. If factory packaging mistakes are made from the Vendor side: Once feedback from Account Manager/BD Dept. Manager has been received, send a task to Purchasing Dept. to ask Vendors to make these revisions if the revisions requested are due to mistakes on Vendor side from mockup to factory packaging.
9. After revisions are made, Design Dept. will send a task with the new sets of factory packaging. BD Salesperson checks and makes sure that all the comments are fixed.
10. After the final factory design has been confirmed by BD Salesperson, send a task to Account Manager and BD Dept. Manager to recheck and provide the final confirmation.
11. If factory packaging requests revisions on our mockup: If revisions required from the Vendors are something to be revised in the mockup, repeat from the previous stage III and revise the packaging mockup. Make sure that new mockup CDR files to be sent to the Vendors are always LOCKED.
12. Once factory packaging has been finalized, do NOT print and sign the final packaging yet. Customer may have some design comments on the packaging.
V. Sending Packaging Design to the Customer and signing Packaging Design Confirmation Agreement (PDCA)
1. BD Salesperson will attach and send the packaging designs in JPG/PDF format to the Customer via email or weTransfer (if files are too big to be sent by email) and ask the Customer to check the mockup design thoroughly. Link to weTransfer is as follows:
2. If the Customer has any reasonable feedback and revision requirements, send the revision requirements to the Design Dept. again via the Batch Number excel sheet.
3. If the Customer has feedback that is not conventional to the standard AdvaCare’s packaging format, politely steer them away from these requests unless it is absolutely a requirement from the MOH for registration. In this case, assess whether the requirements can be made with/without further approval.
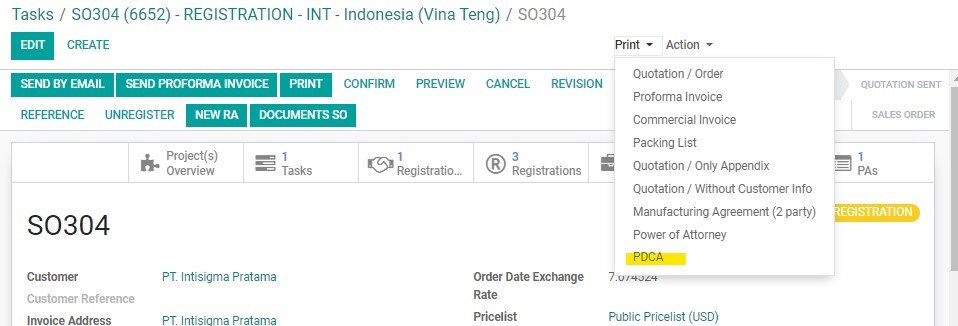
4. Once the Customer has confirmed that mockup design is acceptable, BD Salesperson will prepare the PACKAGING DESIGN CONFIRMATION AGREEMENT (PDCA) . The PDCA template can be exported from the SO page by clicking on “Print” and selecting “PDCA” from the dropdown.
5. Edit the template as per each component of each product and attach the MOCKUP designs accordingly.
6. Make sure that Customer’s signature field is present at the end of every page of the PDCA. Save the PDCA in the following location:
India Purchasing Registration Folder → SOXXXX → PACKAGING DESIGN
7. Send the PDCA to the Customer via email and ask them to sign/stamp each and every page of the PDCA.
8. Once the signed PDCA has been received, set the Registration Milestone Date for REG: 6. Customer Confirms Packaging save in the PACKAGING DESIGN folder with the name format:
VI. Confirming & Signing Final Packaging with the Vendors
1. If Customer did not request any revisions to the mockup as per the latest factory packaging received, skip to step 4 below.
2. If Customer has made revisions to the mockup design, ask Design Dept. to lock the CDRs of the final mockup packaging.
3. Ask Purchasing Dept. to send the final mockup to the Vendors and wait for the final FACTORY PACKAGING that satisfies both Vendor and Customer requirements. The previous steps may be repeated if required.
4. Print out in color every part of each product’s factory packaging. BD Salesperson must write the SO# and date of confirmation on the packaging, sign every page, and provide the printed copies to Design Dept. BD Salesperson must also set the Registration Milestone Date for REG: 7. Manufacturer Confirms Packaging.
5. Design Dept. will scan these copies called “FINAL PACKAGING” and ask Purchasing Dept. to send them to the Vendors as a form of confirmation.