Registration Agreement (RA) refers to an agreement between Vendor and AdvaCare (on behalf of a Customer) to start a registration process of a list of products in a country. In the system, RA is the platform used to keep track of a registration status under this agreement in the ERP system.
Getting started with the RA
Once the RA Specialist is clear on the list of documents and where the documents will come from, they can start to work on the system RA. When opened, the Registration Agreement (RA) page shows an overview the following information for a RA Specialist to check:
GENERAL INFORMATION
The general information of an RA can be found on the main top part of the page. It includes some basic information such as the assigned RA Specialist, SO#, BD Salesperson, Vendor, etc. Some important details to be aware of are:
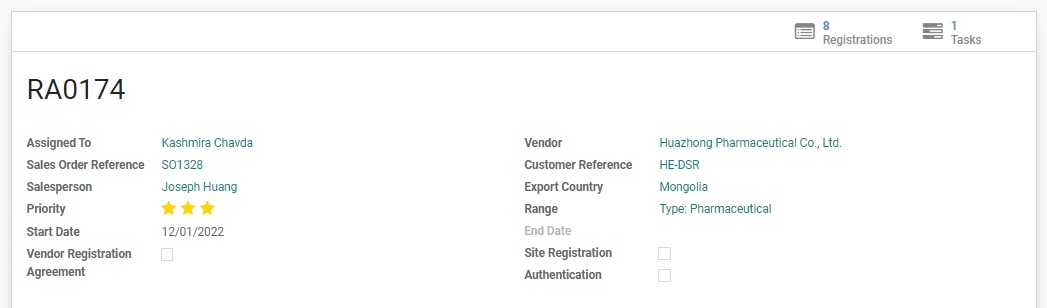
An RA will be assigned a priority level between 1-3, with 3 being the most important. The level of priority is important for RA Specialist to assess which registration to prioritize when there is a backlog of workload.
This checkbox is normally left blank for product registrations.
If this checkbox is ticked, it means that the registration is only for SITE REGISTRATION at the moment. The product list is to be ignored for now. In most cases, registration can go straight to PRODUCT REGISTRATION which is approval of a specific product by submission of the product technical dossiers with some information of the manufacturing site. However, some countries require the approval of the Site first before even starting with product registration.
Shows whether there are any documents that may require Notarization, Legalization, or Apostille for this registration.
IMPORTANT INFORMATION (in the tabs)
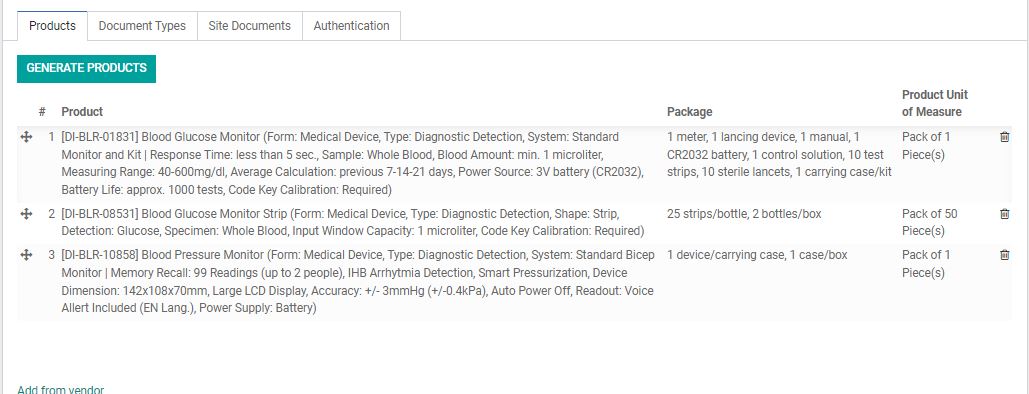
The list of products and its packaging that is to be registered with this Vendor.
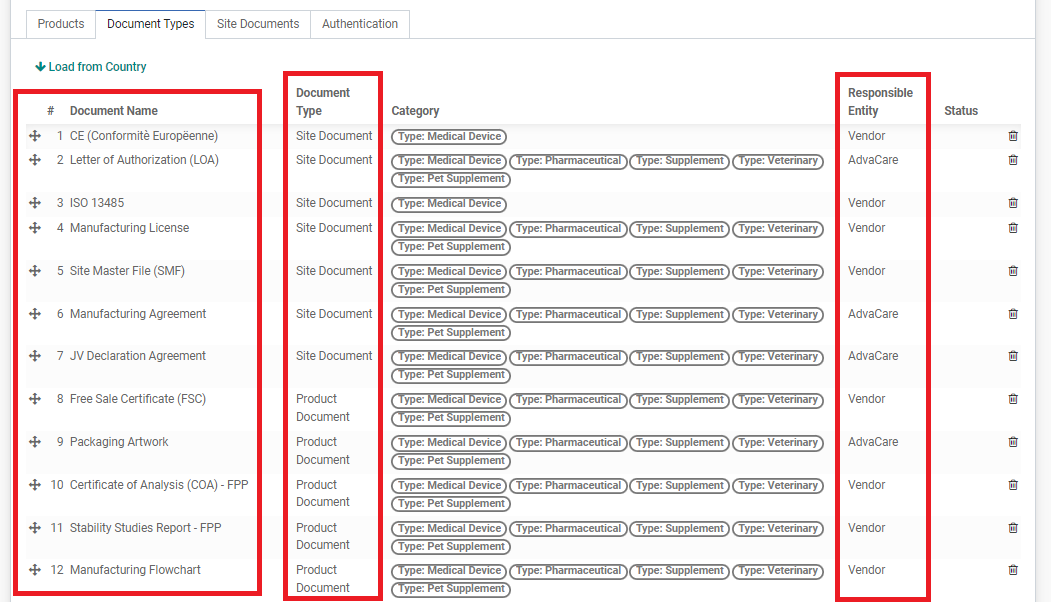
The Document Types tab is the most important part of the RA. It is the list of documents that will be required in this registration, this list is created by BD Salesperson as per the Customer’s list of requirements. The list summarizes:
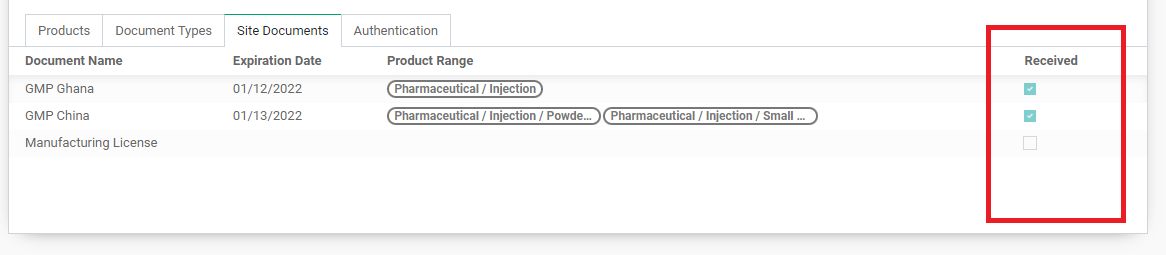
This table tracks the Site Documents, or the quality/technical documents, that the Vendor has confirmed they have for their production site, in relation to the list of documents required from Document Types. It shows the list of site documents that the Vendor has provided, or can provide. There are three ways it will show on the table based on the “Received” checkbox:
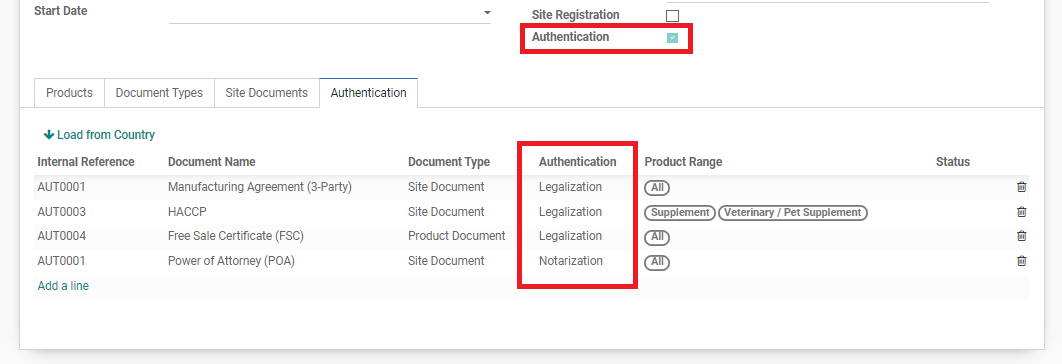
If the “Authentication” checkbox is ticked, it means that some documents will require legalization/notarization. Under the table, a list of documents (Site and Product) that require authentication will be shown. In the column “Authentication” will be the process needed for each document, whether to legalize at the local embassy or to notarize.
After checking all information of the RA, RA Specialists will start working with the Vendor/India Purchasing Person based on the requirements of the RA. If anything is not clear, send a task to BD Salesperson to clarify. If everything is clear, proceed to create Product Registrations (PR).
Checking the RA
Start by checking the following information on the RA:
Cross-check whether the product list and required documents are corresponding. Check as well if the list of documents required under the Handover Task and Task Description are consistent with the document list under the RA.
If there are inconsistencies between requirements mentioned in the task description in comparison to the RA, send a task to BD Salesperson to make the changes. If everything is consistent and RA is ready to be started, proceed to the next part of this SOP.
Moving to the Next RA
If the Registration SO has more than 1 RA (more than 1 Vendor that we are registering with), RA Specialist needs to check the other created RA(s) as well.
Use the breadcrumbs to return to the Task page and locate the other RA(s) from “Opportunity Info” - “Registrations” section again.