After all the information of the RA, Task Description and Handover Tasks are checked and understood by the RA Specialist, a Vendor Registration Agreement (VRA) can be prepared to get started with the registration process. A Vendor Registration Agreement (VRA) essentially is the written agreement that contains the details of an RA for a registration, with the addition of terms and conditions to be agreed upon by both AdvaCare and Vendor before starting with the registration process. There are several purposes to sign the agreement with every Vendor for every new registration:
Confirming the Terms of the VRA
For India registration: Purchasing Dept. will be responsible in finalizing the terms of the VRA including costs and duration of document preparation. RA Dept. can just prepare the draft VRA and send a task to Purchasing Dept. to finalize and sign the VRA with the Vendor. There is no direct communications between India Vendor and RA Specialist.
For China registration: Purchasing Dept. will be responsible in finalizing costs related to the document/sample preparation with the Vendor. However, RA Dept. will be responsible for finalizing the terms of the VRA including the duration of how long the document preparation will take.
RA Specialist can start communicating with the Vendor with an introduction from the Purchasing Dept. For Vendors with prior registrations, RA Specialist can communicate with them directly via QQ or G-mail and reconfirm the following points on the registration:
RA Specialist can send the Vendor Registration Agreement (VRA) draft and send it to the Vendor to confirm.
Preparing VRA
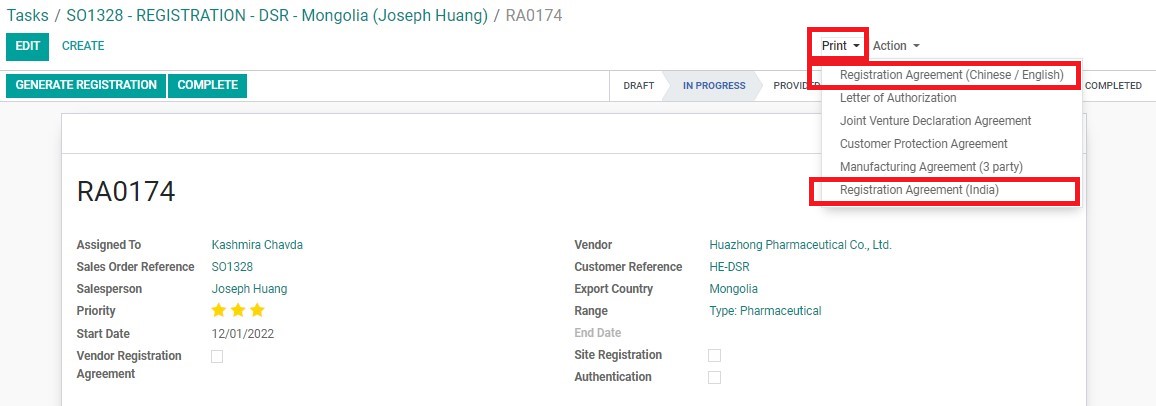
Vendor Registration Agreement can be exported directly from the RA.
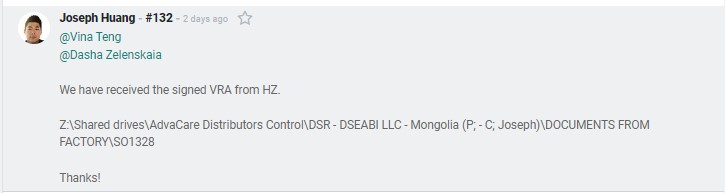
Location: Z:\Shared drives\AdvaCare Distributors Cloud Server\[DISTRIBUTOR NAME]\DOCUMENTS FROM FACTORY
Location: Z:\Shared drives\AdvaCare Purchase India Control\AdvaCare Purchasing\Registration\[REGISTRATION SO#]\DOCUMENTS FROM MANUFACTURER
SO1188 REGISTRATION AGREEMENT - [Factory Name] - signed by both
China registration: Distributor Folder - Documents from Factory and Manufacturer Folder - Agreements
Location: Z:\Shared drives\AdvaCare Distributors Cloud Server\[DISTRIBUTOR NAME]\DOCUMENTS FROM FACTORY
Location: Z:\Shared drives\AdvaCare Cloud Server\Manufacturers\Manufacturers\[MANUFACTURER NAME]\Agreements
India registration: Registration SO Folder - Documents from Manufacturer and Manufacturer Folder - Agreements
Location: Z:\Shared drives\AdvaCare Purchase India Control\AdvaCare Purchasing\Registration\[REGISTRATION SO#]\DOCUMENTS FROM MANUFACTURER
Location: Z:\Shared drives\AdvaCare Purchase India Control\AdvaCare Purchasing\Manufacturers\Manufacturers\[MANUFACTURER NAME]\Agreements
More details on how to update Registration Milestones can be found in the next part of this SOP.