Site Documents Received from the Vendor
| 4 minutesWhen a Site Document is received from the Vendor, it is important that the documents are saved in the Server and also updated in the System. There are a few purposes in this process:
Saving the Site Documents in the Server Folders
Once a copy of a Site Document is received, RA Specialist must check this document, and if relevant to the registration must save it in the Registration Folder - Documents from Factory. If translations will be required, it is important to make sure the translated copy is attached together with the original copy before saving it in the Registration Folder - Final folder.
After that, they must also be saved in the Manufacturer folder - Certificates.
China Manufacturer Folder:
Z:\Shared drives\AdvaCare Cloud Server\Manufacturers\Manufacturers\[Manufacturer Name]
India Manufacturer Folder:
Z:\Shared drives\AdvaCare Purchase India Control\AdvaCare Purchasing\Manufacturers\Manufacturers\[Manufacturer Name]
Vendor site documents should have been saved under the folder “Certificates” and JV Declaration should be saved under “AdvaCare Declaration of Joint Venture Partner”.
Updating “Received” Status in the System
For documents that are newly received, but has already been previously inputted in the System without the “Received” tickmark, RA Specialist must update the status of the documents in the System following this process:
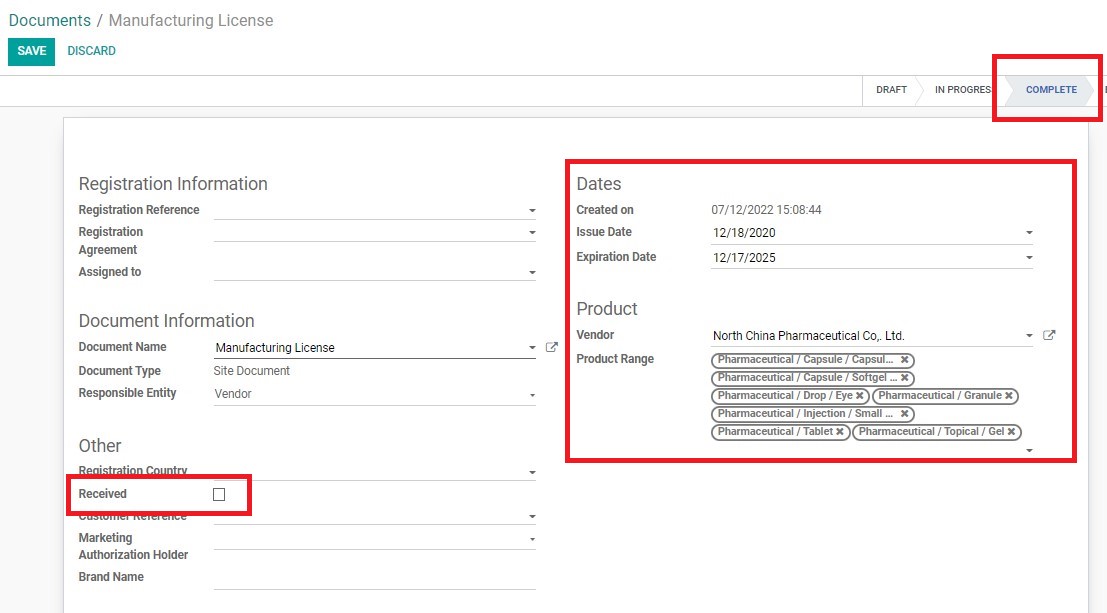
Creating a new Vendor Site Document in the System
