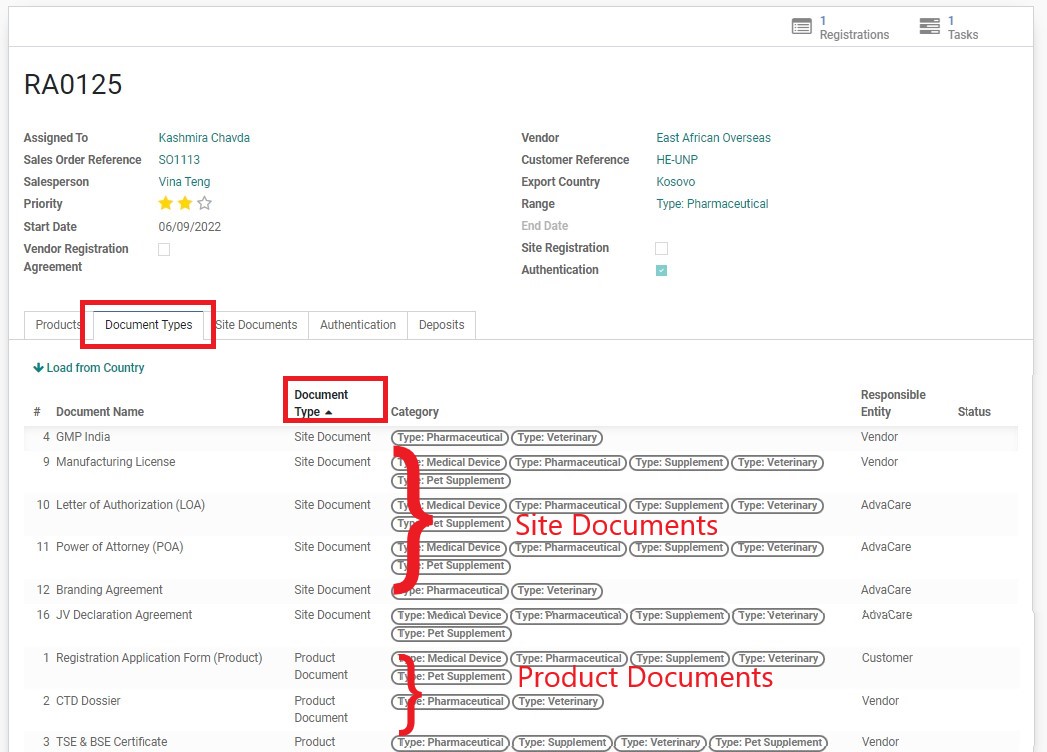
3. Check the list of Site Documents required. Note the responsible entity:
• Responsible Entity: AdvaCare - generally these documents are to be prepared by AdvaCare (BD Salesperson). There are times when some technical assistance will be required from RA Specialist, which BD Salesperson will inform to RA Specialist over task to assist with these documents. Otherwise, they can be ignored.
• Responsible Entity: Customer - likewise, these documents are to be prepared/tracked by BD Salesperson, unless there are tasks to ask RA Specialist’s assistance.
• Responsible Entity: Vendor - these documents are the main Site Documents that RA Specialist will need to gather and track for the registration.
4. Pay attention to the list of Site Documents from Vendor that will be required.
5. Click on the next tab “Site Documents”. This table shows a list of Site Documents from the Vendor that has been inputted into the system. There are three ways to understand these documents:
• Site documents ticked “Received” - documents have already been previously received from the Vendor and saved in the Server.
• Site documents not ticked - document has been confirmed with Vendor that they currently have it, but a copy has not been received.
• Site document is not in the list - Vendor does not currently have this document, or it has not been added to the system yet. If a Vendor Site Document is listed under “Document Types” but not listed here, it means that Purchasing person has confirmed that Vendor can provide these documents and RA Specialist just needs to obtain the certificates from the Vendor directly.
6. RA Specialist can go ahead and start gathering Vendor Site Documents that are already received.
1. RA Specialist can find the “Received ” Vendor Site Document in the Manufacturer Folder.
China Manufacturer Folder:
Z:\Shared drives\AdvaCare Cloud Server\Manufacturers\Manufacturers
India Manufacturer Folder:
Z:\Shared drives\AdvaCare Purchase India Control\AdvaCare Purchasing\Manufacturers\Manufacturers
2. Open the Manufacturer Folder. Vendor site documents should have been saved under the folder “Certificates” and JV Declaration should be saved under “AdvaCare Declaration of Joint Venture Partner”.
Note: Check with Purchasing Specialist responsible for the Registration SO if the certificates cannot be located in the Manufacturer folder even though noted as “Received” in the table. It may have been saved incorrectly in other folders.
3. Open and check the documents to make sure that:
• Description in the certificate matches the name and intended use of the certificate.
• Product range mentioned in the certificates (if applicable), matches with the product list under RA.
• Certificate is still valid.
4. If the documents are accurate and still valid according to the requirements, proceed to save a copy of the certificate in the SO Registration Folder - Final.
5. If the documents are not exactly as per required (for example, applicable only for a different product range from the product list) or already expired, RA Specialist has to ask for the new correct certificate from Vendor. Follow the next SOP.