General Documents Handbook
| 12 minutesGENERAL DOCUMENTS
Below you can find the general description of documents usually required for registration.
GOOD MANUFACTURING PRACTICE. Good manufacturing practice (GMP) is a system for ensuring that products are consistently produced and controlled according to quality standards. It is designed to minimize the risks involved in any pharmaceutical production that cannot be eliminated through testing the final product.
GMP covers all aspects of production from the starting materials, premises, and equipment to the training and personal hygiene of staff. Detailed written procedures are essential for each process that could affect the quality of the finished product. There must be systems to provide documented proof that correct procedures are consistently followed at each step in the manufacturing process - every time a product is made.
GMP certificate is a document issued by a government or government health authorities after conducting an audit (inspection) of the manufacturing facility. GMP certificate is a document that proves that the manufacturer conforms and follows GMP standards.
Every country that enforces GMP requirements conducts periodical inspections of the local manufacturers and after inspection is passed, issues GMP certificate. If inspection is not passed, the manufacturer has to apply for re-inspection and is not allowed to run production until the GMP certificate is granted. Similarly, foreign manufacturers that want to distribute their products in the country that enforces GMP requirements have to get GMP clearance from this country. GMP certificate is valid for a certain period, after which re-inspection needs to be arranged otherwise distribution in this country has to stop.
Different countries have different GMP requirements. The WHO version of GMP is used by pharmaceutical regulators and the pharmaceutical industry in over 100 countries worldwide, primarily in the developing world. The most strict GMPs are enforced in the USA, with the EU and the UK following closely behind. Similar GMPs are used in other countries, with Australia, Canada, Japan, Saudi Arabia, Singapore, Philippines, Vietnam and others having highly developed/sophisticated GMP requirements. If a manufacturer wants to get their products distributed in one of the above countries, they need to pass an audit (inspection) conducted by drug authorities of that particular country.
Developing countries also follow GMP standards, however, their requirements are not as strict. Some developing countries require inspection, others don't. Some countries MOH accept GMP certificates from one of the developed countries instead of conducting their own inspection.
Customers might require GMP certificates from different countries as it helps them to register faster. Few countries can accept only high level GMP certificates, so Salesperson has to confirm with the Customer if Indian/Chinese GMP certificate is enough or other countries GMP certificates will be required.
High level types of GMP:
- EU-GMP - GMP issued by European Medicines Agency of the European Union.
- MHRA - GMP issued by the Medicines and Healthcare Products Regulatory Agency of the United Kingdom.
- USFDA - GMP issued by the United States Food and Drug Administration.
- TGA - GMP issued by the Therapeutic Goods Administration of Australia.
HACCP. Hazard Analysis Critical Control Point system is a management system in which food safety is addressed through the analysis and control of biological, chemical, and physical hazards from raw material production, procurement and handling, to manufacturing, distribution and consumption of the finished product.
This certificate can be termed as GMP for supplements products. Supplements products are considered as Food products and their quality certificate is HACCP.
CE. Conformitè Europëenne (CE) Certificate is the certificate that indicates conformity with health, safety and environmental protection standards for products sold within the European Economic Area (EEA). CE certificate applies to Medical devices and PPE products.
The CE marking is the manufacturer's declaration that the product meets EU standards for health, safety, and environmental protection. The mark consists of the CE logo and, if applicable, the four digit identification number of the Notified Body involved in the conformity assessment procedure. CE marking is mandatory for Medical Devices and PPE products entering EU market, and it is required by some other countries.
Salesperson has to check with the Customer if CE certificate is required. If it is, Salesperson has to inform Purchasing Dept. in the initial RFQ to quote from the manufacturers which are CE certified.
ISO. The International Organization for Standardization is an international standard-setting body composed of representatives from various national standards organizations. ISO is a standard mark which can be on all the products. Usually clients will ask for this certificate only for Medical Devices. Salesperson has to check with Customer if ISO certificate is required. If it is, Salesperson has to inform Purchasing Dept. in the initial RFQ to quote from the manufacturers which are ISO certified.
Many times Customers can accept AdvaCare ISO certificate although AdvaCare ISO certificate number is different as it is not for the manufacturers.
COPP. The Certificate of Pharmaceutical Product establishes the status of the pharmaceutical product and of the applicant for this certificate in the exporting country. It is issued for a single product, because manufacturing arrangements and approved information for different pharmaceutical forms and strengths can vary. COPP is needed by the importing country when the product in question is intended for registration. In China COPP is issued by FDA, and product has to be registered in the local market to get COPP. If product is not registered in the local market, it is very difficult to get COPP. Salesperson has to check with Customer if COPP is required. If it is, Salesperson has to inform Purchasing Dept. in the initial RFQ to quote from the manufacturers that can provide COPP for the required products.
FSC. The Certificate of Free Sale is evidence that goods, such as food items, medicines and medical devices are legally sold or distributed in the open market, freely without restriction, and approved by the regulatory authorities in the country of origin. This certificate is issued to manufacturers by the government authority. Salesperson has to check with the client if FSC certificate is required. If it is, Salesperson has to inform the Purchasing Dept. in the initial RFQ to quote from the manufacturers which are FSC certified.
ML. Manufacturing License is a license granted by the Regulatory Authority to the manufacturer in the applicable territory to manufacture the licensed product. This is a very basic document and all the manufacturers have this certificate.
LOA. Letter of Authorization is a letter in which one person or business grants another person or business the authority to act on their behalf. Customers require Letter of Authorization to register products in the importing country on behalf of AdvaCare.
Letter of Authorization authorizes the Customer to register/market/distribute AdvaCare products.
There are situations when Customer cannot accept the LOA from AdvaCare and needs it from the actual manufacturer. In this case Salesperson needs to prepare the LOA from the manufacturer name and send a task to Registration Dept. to get it stamped and signed.
COA. A Certificate of Analysis is a document issued by Quality Assurance that confirms that a regulated product meets its product specification. They commonly contain the actual results obtained from testing performed as part of quality control of an individual batch of a product.
It is a very common document and can be prepared in house by getting all the relevant information from the manufacturer.
Stability Study. Stability studies are performed to determine the effects of environmental conditions on product quality. Stability studies provide the supporting data that companies use to establish product storage requirements and expiration dating. Manufacturers usually have this document and if Customer requires it Salesperson has to ask Registration Dept. to check with the manufacturer and get it. However, there are different types of stability studies. Some of the countries with hot climate require stability studies performed at higher temperatures to make sure that product is stable in their environmental conditions. This type of stability studies is less common.
Stability studies is also a part of CTD dossiers.
SMF. Site Master File (SMF) is a document in the pharmaceutical industry which provides information about the production and control of manufacturing operations. Manufacturers usually have this document and if Customer requires it Salesperson has to ask Registration Dept. to check with the manufacturer and get it.
DOSSIERS
A dossier is a collection of papers or other sources, containing detailed information about a particular person or subject. A dossier can be an entire folder of pertinent information regarding a product or subject. That means a variety of reports can make up the contents. It is a detailed project and takes time to complete as it involves reports of the product which are taken over years.
CTD dossiers. The Common Technical Document (CTD) is a set of specifications for an application dossier for the registration of Medicines. It is an internationally agreed format for the preparation of applications regarding new drugs intended to be submitted to regional regulatory authorities in participating countries.
The Common Technical Document is divided into five modules:
- Administrative and prescribing information;
- Overview and summary of modules 3 to 5;
- Quality (pharmaceutical documentation);
- Preclinical (Pharmacology/Toxicology);
- Clinical – efficacy and safety (Clinical Trials).
CTD dossiers are provided for the Pharmaceuticals, Veterinary products and Supplements.
CTD dossiers are chargeable. If the Customer mentions that he needs CTD dossiers, check with him first if this document is absolutely required and if so, then if all the 5 modules are required.
ECTD dossiers. Electronic Common Technical Document (eCTD) is the electronic version of CTD. This is the new technique of submitting dossiers that has recently come up. Many countries MOH prefer eCTD dossier. The formats of CTD dossiers and ECTD dossiers are a little different, but the content is the same. It is important to ask the Client to confirm whether they need CTD or eCTD.
ACTD dossiers. Asean Common Technical Document (ACTD) consists of Parts I to IV:
Part I : Table of Contents, Administrative Data and Product Information;
Part II : Quality Document;
Part III : Nonclinical Document;
Part IV : Clinical Document.
This type of dossier is accepted by most of Asian countries. If the Customer is from any of the Asian countries, double check with them if ACTD dossiers are accepted.
FWA dossiers. French West Africa (FWA) dossiers are much easier dossiers and do not cover detailed clinical studies. These dossiers are accepted only by the French African countries. If the Customer is from any of the French African countries, Salesperson has to confirm if FWA dossiers are required.
Find the content of the FWA dossier below:
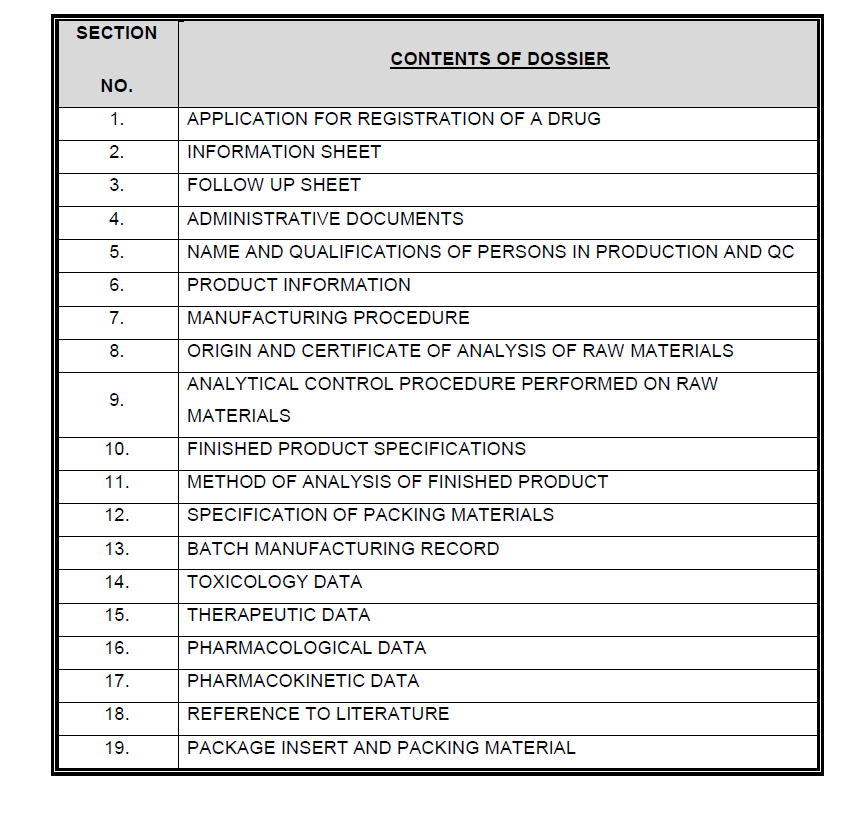
STED dossiers. Summary Technical Documentation (STED) are the dossiers prepared for medical devices. This dossier involves all the detailed technical information related to the medical device. STED are not very strict and are easy to prepare.
Components of STED dossier:
- Device description;
- Device history;
- Essential Principles checklist;
- Risk analysis and control summary;
- Design and manufacturing information;
- Clinical evidence report;
- Performance evaluation;
- Product validation and verification;
- Stability;
- Information to be supplied with the Medical Device.
After it is confirmed with the Customer which type of dossier is required for registration, Salesperson has to pass the information of all registration requirements to the Registration Dept. Registration Dept. will check if factories can provide the documents, or if dossiers can be prepared by AdvaCare in house. If neither is possible, Salesperson has to send a task to Business Development Supervisor to get the cost of preparing the dossier with the agent. After the cost is received, Salesperson has to inform the Customer of all the registration costs.
BE STUDIES and DISSOLUTION PROFILE
Bioequivalence (BE) studies are a surrogate marker for clinical effectiveness and safety data, as it would not normally be necessary to repeat clinical studies for generic products. For oral drugs, it is accepted that if blood concentrations of the active ingredient of the generic and brand name drugs are the same, then their concentration at the site of action and therefore their safety and effectiveness will also be the same. For other dosage forms (e.g., drugs for inhalation, topical, or parenteral use), bioequivalence can be demonstrated through other comparative testing (e.g., comparative pharmacodynamic studies, pharmaceutical properties) in addition to or in lieu of comparative bioavailability to support the safety and efficacy of the proposed product.
For oral drugs, bioequivalence is determined by comparing the relative bioavailability of the brand name drug versus the generic drug.
Generally most of the Customers do not need BE studies. The original BE studies are very expensive and come only from the certified laboratories. AdvaCare does not provide these studies to clients.
The other type of BE study is done by the small laboratories which are not authenticated but can provide the accurate data. These BE studies are much cheaper than the original ones. Salesperson has to check with the client if they need BE studies and if so, send a task to the BD Dept. Supervisor to get the cost from the agent.
After getting the cost, Salesperson has to inform the Customer along with other registration costs.
Dissolution Profile is a standardised method for measuring the rate of drug release from a dosage form, it is essential that all the apparatus used for the testing produces the same sets of results given all other parameters are equal. This process is done by dissolving the drug.
A lot of countries MOH can accept Dissolution Profile in place of BE study as it also provides the complete data related to the drug, it’s usage and effects.
Dissolution study is much cheaper than the BE study.
After the Customer informs Salesperson that BE study is required, Salesperson has to check if Dissolution Profile can be accepted instead of BE study. If so, Salesperson has to send a task to BD Dept. Supervisor to get the cost from the agent. After getting the cost, Salesperson has to inform the Customer along with other registration costs.
DRUG MASTER FILE
Drug Master File (DMF) is a document prepared by a pharmaceutical manufacturer and submitted solely at its discretion to the appropriate regulatory authority in the intended drug market.
Usually all the manufacturers have DMF. When DMF is required, Salesperson has to check with the Registration Dept. to confirm if the manufacturer can provide it.
If the manufacturer for some reason cannot provide DMF, Salesperson must send a task to BD Dept. Supervisor to get the cost from the agent. After getting the cost, Salesperson has to inform the Customer along with other registration costs.